Materials International, 2019, 1, 1, 0013-0024, https://doi.org/10.33263/Materials11.013024
Mădălina Elena David1,*, Alexandru Mihai Grumezescu2
- National Research & Development Institute for Chemistry and Petrochemistry–ICECHIM, 202 Splaiul Independentei, Sector 6, Bucharest 060021, Romania
- Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Materials Science, Politehnica University of Bucharest, 060042 Bucharest, Romania
* Correspondence: madalina.e.david@gmail.com
Abstract: In recent years, nanomedicine focused on the development of functional AuNPs for biomedical imaging, attributed to the intriguing optical properties of these nanoparticles, which are discussed in this review. Moreover, are presented the most important in vivo diagnostic techniques which have benefited from the development of engineered AuNPs, such as computed tomography and photothermal/photoacoustic imaging. Another important advantage related to these nanoparticles refers to their excellent performance in recent in vivo studies and clinical trials. Also, side effects of conventional drugs have been minimized by conjugation of AuNPs.
Keywords: gold nanoparticles, biomedical imaging, biocompatible nanoparticles.
Abbreviations: DOX: doxorubicin, PVP: Polyvinyl pyrrolidone, Hyd: Hydrazone, PEG: Polyethylene glycol, BLM: Bleomycin, CPP: Cell penetrating peptides, MTX: Methotrexate, 3-MPA: 3-mercaptopropionic acid, TAM- tamoxifen, FA: Folic acid, BHC: Berberine hydrochloride, Gem: gemcitabine, C225: cetuximab, DOD: dodecylcysteine, LL2: Lewis lung carcinoma, EAC: Ehrlich-Lettre ascites carcinoma.
| © 2019 by the authors. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). |
1. Introduction
Presently, numerous nanoparticles and nanomaterials synthesized either biologically or physiochemically have emerged from different bulk elements such as gold, silver, iron, copper, in order to be used in advanced nanotechnology and medical science [1, 2]. One of the main advantages of these nanoparticles is represented by the ability to control their properties (physical, chemical and biological), which offer many possibilities to explore these nanoparticles in applications like drug delivery, as image contrast agents and for diagnostic purposes [3]. In comparison with others nanoparticles, gold nanoparticles (AuNPs) offer unique optical and Surface Plasmon Resonance (SPR) properties, which make them suitable to be used in biological and pharmaceutical fields, such as imaging-based therapeutic techniques and ultrasensitive detection for the treatment of cancer [4, 5].
Cancer is caused by abnormal cell growth and is the second leading cause of death globally, being responsible for an estimated 9.6 million deaths in 2018. According to the World Health Organization (WHO) about 1 in 6 deaths is due to cancer [6]. Presently, the treatment of cancer is based on chemotherapeutic drugs, with the aim of killing the cancer cells. It has been demonstrated in several studies that these treatments often result in side effects due to the damage caused to the surrounding healthy tissues [3].
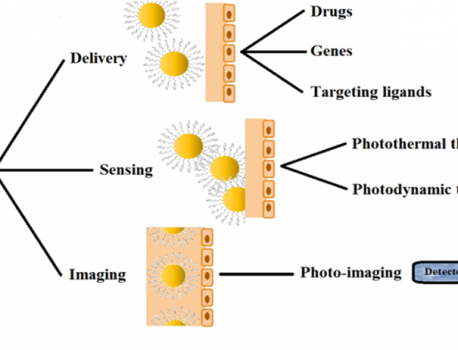
In the last several years, AuNPs (bare or functionalized) have received important attention in nanotherapeutic cancer treatment (Figure 1) due to their unique properties, which make them suitable candidates for conjugation with targeting ligands, imaging labels, and therapeutic drugs. Also, it has been demonstrated that functionalized AuNPs can be used for targeted molecular imaging and localized surface plasmon resonance (LSPR) [7-9].
There are two processes are involved in differentiating malignant and nonmalignant cells: passive targeting and active targeting. Passive targeting takes advantage of the enhanced permeability and retention (EPR) effect observed in tumors to increase the concentration of AuNPs. The second process involves the selective molecular recognition of antigens that are expressed on the surfaces of cancer cells to localize AuNPs to malignant cells or the exploitation of the membrane properties associated with malignancy [10, 11].

Typically, AuNPs are defined as particles of 1–100 nm in size, which is in the sub-wavelength regime of visible light [12, 13]. These nanoparticles with controlled size and shape are synthesized by various physical, chemical, and biological ways [3].
Physical methods refer to the energy transfer that occurs in a material when it is irradiated using ionizing or non-ionizing radiation, which may trigger the reduction reactions that lead to the nucleation of metallic particles. This method includes photochemical processes, ionizing radiation and microwave radiation [14-16]. Ngo V.K.T. and co-workers obtained AuNPs by a low cost technique, microwave heating in order to investigate the effect of different elements (precursor reagents, irradiation time, and microwave radiation power) on the morphology of AuNPs. It was observed that the size of AuNPs decreased and the size distribution became narrower with increasing the concentration of sodium citrate. Also, it has been reported that a longer reaction time and higher microwave radiation power increased the NPs size, demonstrating that microwave heating had a strong effect on the yield of the AuNPs [17]. Zhou Y. and co-workers obtained shape-controlled AuNPs by a novel ultraviolet irradiation technique at room temperature. It was demonstrated that, not only the concentration of Au cations and the irradiation time influenced the morphology of AuNPs, but also the concentration and the species of the polymer capping materials play an important role. The prolongation of irradiation time facilitated the formation of the AuNPs with more regular shape [18]. In another study, it was demonstrated that the production of hexagonal AuNPs began within seconds of microwave irradiation and the size growth increased with the microwave power and time [19].
Chemical methods utilize chemicals and solvents, like sodium borohydride (NaBH4), hydrazine and citrate to initiate the synthetic process and promote nanoparticle nucleation. It has been demonstrated that the most efficient reducing agents are NaBH4 and hydrazine, but these agents present the disadvantage that are biologically and environmentally toxic [20-23]. Suchomel P. and co-workers prepared AuNPsby the reduction of tetrachloroauric acid using maltose in the presence of nonionic surfactant Tween 80 at various concentrations, in order to control the size of the resulting AuNPs. It was observed that when the concentration of Tween 80 increased, a decrease in the size of produced AuNPs was observed, which ment that the surfactant plays a key role in the nanoparticles dimensions [24].
Biological synthesis (plants and microorganisms mediated) is a relatively new, eco-friendly, and promising area of research. Presently, it has been demonstrating that numerous medicinal plants have shown potential to produce stable AuNPs [25-27]. There are some advantages using this method for making AuNPs, such as nontoxic biocomponents, limiting the waste formation and cutting down the need for extra purification steps. This method involves mixing the gold salt with extracts of plant under varied reaction conditions like pH, incubation time and temperature to obtain specific shapes and sizes of AuNPs [18]. Among various methods, chemical reduction of Au3+ ions is considered to be the best method to synthesize AuNPs with controlled size and morphology [28, 29].
In the last several years, these various synthesis methods to obtain AuNPs have become an attractive and potential option to explore as a tool for photothermal therapy (PTT), photodynamic therapy (PDT), photoimaging, targeted drug delivery, and immunoassays. Presently, various types of AuNPs, such as gold nanorods, nanocages, nanostarsand nanospheres, have become effective tools in human cancer [7, 30, 31].
In this review, we focus on providing further new insights for exploring the AuNPs applications as a tool in cancer imaging and therapy.
2. Gold Nanoparticles in Cancer Imaging
AuNPs received significant attention due to their high absorption coefficient, potential biocompatibility and relatively low toxicity. Also, it is very important for AuNPs to be synthesized under special conditions which can reduce concerns regarding the potential toxicity induced by the reducing agents and reaction conditions [32].
The main advantages of AuNPs in imaging applications are related to the fact that:
- AuNPs have long body circulation times;
- The AuNPs selectively accumulate at sites of interest through the enhanced permeability and retention (EPR) effect or by surface modification with specific coatings;
- AuNPs present a large absorption in the near-infrared window for photothermal therapy;
- Their simple functionalization [33].
Due to the high rate of cancer patients around the world, the development of new techniques to diagnose early cancers is essential. The use of AuNPs offer long-time operation for optical imaging, because of the properties of these nanoparticles (i.e. photoresistance, stability). Also, these nanoparticles are efficient contrast agents in optical imaging as a result of their unique interaction process with light particles. The most important in vivo diagnostic techniques are: i) computed tomography (CT), ii) photothermal/ photoacoustic imaging, iii) two-photon fluorescence imaging, iv) optical coherence tomography (OCT), v) Raman spectroscopy and vi) light scattering imaging [32]. Typically, AuNPs with dimensions between 30 and 100 nm scatter intensely and can be easily detected using a commercial microscope under dark-field illumination conditions [34]. It has been reported that the scattering cross-sections of AuNPs are 105–106 times stronger than the emission from a fluorescent dye molecule [35].
2.1. Gold Nanoparticles used in CT
Computerized tomography (CT) allows obtaining of 3D anatomic imaging at a high spatial resolution by using the difference in the absorption effect from different human tissues. Also, this technique uses an X-ray contrast agent which has the role to distinguish tissues with similar or low X-ray attenuation. CT is a valuable medical tool and it is used in several applications, such as diagnose, monitoring of the therapy effectiveness (e.g. for cancer treatment) and blood pool imaging [36-38].
AuNPs have gained recent attention as an X-ray contrast agents for CT imaging due to some important features like the higher atomic number and the electron density of gold, but also the feasibility of AuNPs to enhance the in vivo vascular contrast in CT imaging and the capacity of AuNPs to selectively target tumor specific antigens [32, 34, 39-42]. AuNPs with average diameter of 1.9 nm were used as contrast agent for CT to detect tumors in mice. It was reported that after 24 hours, the AuNPs were not detected in the blood, but showed significant accumulation after 15 min in the kidney, tumor tissue, liver and muscle. It was reported that these nanoparticles were cleared by renal excretion and were not found in liver or spleen [43]. In another study, PEG-coated AuNPs were used to impart antibiofouling properties to extend the systemic circulation half-life. These nanoparticles were injected intravenously into rats and their longer blood circulation time (4 h) was observed by CT in case of PEG-coated AuNPs, as compared with an iodine contrast agent (<10 min) [44]. Also, PEG-coated AuNPs injected in adult Balb/c mice which underwent microcomputed tomography scans revealed a good biocompatibility without toxicity marks in tested mice. A stable imaging window for visualizing the vasculature system, at time zero (immediately after injection and up to 24 hours after injection) was obtained [45]. In another study, AuNPs obtained by encapsulating gold cores within a multilayered gadolinium chelates bound organic shell, were used as contrast agents for both in vivo X-ray and MRI (Magnetic Resonance Imaging). It was reported that these particles are suitable for dual modality imaging and freely circulate in the blood vessels without undesirable accumulation in the lungs, spleen, and liver due to the fact that the contrast enhancement in MRI stems from the presence of gadolinium ions which are entrapped in the organic shell, whereas the gold core provides a strong X-ray absorption [46].
Zavaleta C.L. and co-workers reported a new study that consisted of a synthesis of two types of AuNPs, namely porous AuNPs (PAuNPs) and solid AuNPs (SAuNPs). The first set was prepared by reduction of a gold solution with lecithin, whereas the second set was fabricated by a reflux method using lecithin as a single reducing agent. CT scanning revealed that PAuNPs presented a brighter contrast (45 HU) than SAuNPs (26 HU). In vivo tests were carried out on male rabbits which were intravenously injected with 1 mg/kg weight of PAuNPs/SAuNPs. After 72 hours, it was observed that PAuNPs showed an enhanced contrast compared to SAuNPs 6 hours after injection in organs scanned by CT. It was reported that IV administration of synthesized PAuNPs increased the levels of aspartate aminotransferase (AST), alkaline phosphate (ALP), serum creatinine, and blood glucose, whereas that of SAuNPs increased the levels of AST, ALP, and blood glucose [47].
A new study revealed that glucose-functionalized AuNPs can be used as a metabolically targeted CT contrast agent. It was reported that, due to the unique characteristics of tumor vasculatures and dissimilarities between cancer and inflammatory processes, these complexes accumulate in the tumor and not in the inflammatory lesion, thus preventing false-positive results [48]. In comparison with other techniques (such as MRI, positron-emission tomography), current X-ray imaging provides lower sensitivity and no ability for molecular imaging. So, a new and optimized X-ray contrast agent is required to give patients these benefits, AuNPs being a promising candidate [38].
2.2. Gold Nanoparticles used in Photothermal/Photoacoustic Imaging
Photothermal/photoacoustic imaging refers to the laser-induced heating of materials, with the former relying on the direct detection of heat and the latter on the detection of acoustic waves generated by the thermal expansion of air surrounding the materials. In biomedicine, photoacoustic imaging is used more frequently than photothermal imaging, due to the fact that the photoacoustic technique combines the high contrast of optical imaging and the deep tissue penetration of ultrasound imaging [33]. There are several key features to consider when the particles are selected for hyperthermia, such as the wave-length of maximal absorption, the absorption cross-section, and the size of the particle [49].
AuNPs are the main mediators of photothermal therapy because of several advantages, such as biocompatibility, efficient light-to-heat conversion, small diameters that enable tumor penetration upon systemic delivery, simple gold-thiol bioconjugation chemistry for the attachment of desired molecules, and the ability to be tuned to absorb near-infrared (IR) light, which penetrates tissue more deeply than other wave-lengths of light [50].
Presently, several forms of gold-based nanoparticles have been developed, such as gold–silica nanoshells, colloidal gold nanospheres, gold nanorods, and smaller-diameter near IR (NIR) -tunable gold nanocages [49].
Recently, a novel photo-cross-linkable AuNP was developed. It was observed that the surface plasmon resonance peak of 20.5 nm AuNPs can effectively be shifted to NIR regions, which makes small AuNPs not only useful for enhanced photoacoustic imaging, but also for effective photothermal therapy of malignant tumors [51]. In another study, photothermal imaging was used to detect prostate cancer by using AuNPs conjugated with anti-HER2 [52].
Yang Z. and co-workers fabricated a complex by self-assembly of poly(perylenediimide) (PPDI) and PEG tethered AuNPs (Au@PPDI/PEG). It was reported that the PPDI offered a greater photothermal effect and the resulted complex proved excellent therapeutic and in vivo biomedical imaging potential [53]. Also, AuNPs were used to observe the circulation of these nanoparticles in real-time, in blood vessels in the neck region of a mouse injected [54].
The use of AuNPs in photothermal/photoacoustic imaging offers a more accurate imaging of the tumor and moreover, these nanoparticles play an important role in photothermal imaging- assisted treatment modalities [55].
2.3. Gold Nanoparticles used in Raman spectroscopy
Raman spectroscopy is a sensitive method based on inelastic scattering of light by vibrating molecules and can provide various biochemical informations about cells, tissues or biofluids [56, 57]. The size and surface charge of surface-enhanced Raman scattering (SERS) nanoparticles influence their in vivo biodistribution and thus, may affect the efficacy in raman spectroscopy. AuNPs without a passivating shell, also have the tendency to aggregate in vivo, but several studies reported that this agglomeration can be prevented by applying silica or PEG coatings [58].
Also, AuNPs can be used to prepare SERS nanoparticles for small animals. Raman spectroscopy and AuNPs with a silica coatings were used to separate the spectral fingerprints of up to 10 different types of SERS nanoparticles in a living mouse and to colocalize five different SERS nanoparticles within deep tissues after intravenous injection [59]. In another study, it was reported a new and facile strategy for the DNA-assisted decoration of single-walled carbon nanotubes (SWNTs) with AuNPs and their application in SERS imaging. The complex formed with SWNT-AuNPs was functionalized with synthetic DNA, in order to obtain nanocomposites with enhanced Raman signal. The big advantages of the proposed method were the presence of the free DNA overhangs around the SWNT-AuNPs which made the final nanocomposites a promising candidates in selective cancer cell labeling and imaging, or sensor developments [60]. It was reported that AuNPs-based SERS agents can improve the patient safety by highlighting the tumor margins to help ensure the complete removal of residual diseased cells and to avoid surgical injury of the normal tissues[61].
2.4. Gold Nanoparticles in Cancer Therapy
AuNPs have been gaining popularity in medical applications for several reasons. One reason is given by their potential to be relatively non-reactive in biological environment, character which makes them suitable for in vivo applications. Moreover, properties like strong optical behaviour, easy controllable surface chemistry enabling versatility in adding surface functional groups and ease in control over particle size and shape during synthesis contribute to AuNPs esteem. Due to these reasons, AuNPs are considered to be fully multifunctional and offer the possibility of combining different desired functionalities in one molecular-sized package [62-64].
2.5. Gold Nanoparticles as drug delivery agents targeted to cancer cells
Presently, chemotherapy is the most used method for the treatment of cancer, even if it presents many limitations, mainly due to the numerous side effects resulting from non-specific interactions of drugs with cells and tissues, and low solubility. So, because of these important disadvantages, it is very important to improve the existing therapies. One method refers to the use of drug delivery systems which could provide efficient targeted transport and overcome limitations of standard anticancer therapy. These systems have to be capable of stocking an adequate amount of drug, bypassing mechanisms of drug resistance, improving biodistribution and preventing fast removal of the drug from the body. Also, these systems should be performed with prolonged biological half-life, tumor accumulation, efficient cellular uptake and controlled release patterns. In several studies, it has been reported that AuNPs present the ability to meet almost all of the above requirements [34, 62, 65-68].
Table 1. Drugs conjugated with AuNPs.
| Nanoparticle | Nanoparticle Size (nm) | Cell Lines | Remarks | Ref. |
| DOX@PVP-AuNP | 12 | A549, H460, and H520 human lung cancer cells | Induction of early apoptosis in lung cancer cells and upregulation of tumor suppression genes. | [70] |
| DOX-Hyd@AuNP | 30 | MCF-7/ADR cancer cells | Enhanced toxicity against multi drug resistant cancer cells. | [71] |
| DOX-BLM-PEG-AuNP | 10 | HeLa Cells | Enhanced half-maximal effective drug concentration. | [72] |
| CPP-DOX-AuNP | 25 | HeLa cells and A549 cells | Higher cell death. | [73] |
| DOX-PEG-AuNP | 12 | KB cellsand A549 cells | Higher cytotoxic effect as compared to free DOX. | [74] |
| MTX-AuNP | 8-80 | LL2 cells | Higher cytotoxicity towards numerous cell lines as compared to free MTX. | [75] |
| 3-MPA-AuNP | 5 | K562/ADM cells | Higher cell death. | [76] |
| TAM-PEG-AuNP | 25 | MCF-7 and HSC-3 | Higher cell death. | [77] |
| FA-BHC-AuNP | 20-60 | Vero and HeLa | Increased efficacy of BHC against cancer cells. | [78] |
| Gem-C225-AuNP | 5 | PANC-1, AsPC-1, and MIA Paca2 | Significant inhibition of pancreatic tumor cell proliferation. | [79] |
| DOD-AuNP | 3-6 | EAC | Anti-tumour activity of the prepared surfactant was enhanced with the presences of the AuNPs. | [80] |
It has been reported that AuNPs present the following advantages as drug delivery systems:
- Large surface area- they offers high loading capacity of drug, improving the drug hydrophilicity and stability (AuNPs can be synthetized in a large board of sizes, from 1 to 150 nm);
- Easy surface modification, with targeting ligands to enhance the tumor selective accumulation (the presence of a negative charge on the surface of AuNPs makes them easy to be modified, they can be functionalized easily by the addition of various biomolecules such as drugs, targeting ligands, and genes);
- Passive targeting ability to tumor site, due to their leaky neo-vessels (EPR effect);
- Controlled release of drugs, in the case of internal or external stimulus [7].
Over the last several years, AuNPs have been conjugated to a variety of antitumor substances, either hydrophobic or hydrophilic. For example, 5-Fuorouracil was attached to AuNPs with terminal carboxylic acid groups from the capping agent, in order to investigate their potential anticancer effect. Ma X. and co-workers synthesized SM5-1 (humanized mouse monoclonal antibody)- conjugated AuNPs and investigated their anticancer efficacy in hepatocellular carcinoma (HCC) both in vitro and in vivo. The studies proved that conjugation of SM5-1 and AuNPs efficiently increased the tumor growth inhibition rates in HCC. In addition, the bioluminescent images showed that AuNPs- SM5-1 can achieve considerable antitumor efficacy in HCC, providing a potential therapy approach for HCC [69]. Other drugs conjugated with AuNPs are summarized in Table 1.
3. Biodistribution and Toxicity Aspects of Gold Nanoparticles
AuNPs have been extensively explored in biomedical applications, especially as drug carriers or contrast agents. However, AuNP can exhibit a cytotoxic profile, when the size of the nanoparticles is below 2 nm (ultrasmall AuNP) or when the stabilizing ligands determine a direct interaction with biomolecules or for catalytic activity of the unshielded gold surface. It has been reported that these ultrasmall AuNPs exhibit significantly different biodistribution and enhanced circulation times compared to larger AuNPs [81-85].
Also, in several studies it has been reported that anticancer drugs conjugated with AuNPs exhibited higher cytotoxicity towards numerous tumor cell lines, compared with the equivalent free anticancer drug. For example, MTX was used to treat cancer for decades, but upon conjugation with AuNPs displayed higher cytotoxicity towards numerous tumor cell lines as compared to that of free MTX. MTX was observed to accumulate in the tumor cells at a faster rate and to a higher level when conjugated with AuNPs [75]. Also, DOX bounded to AuNPs via an acid labile linker, showed enhanced toxicity against the multi drug resistant MCF-7/ADR breast cancer cell line, thus overcoming the multi drug resistance to some extent due to the enhanced uptake of the AuNPs-tethered drug followed by its responsive release within the cell [71].
The in vivo biodistribution of AuNPs was intensively studied. For example, Le Q. L. and co-workers evaluated the in vivo distribution of AuNPs (20 nm) after intravenous administration in mice. It was observed that after 1 hour of administration, the nanoparticles were mainly accumulated in blood (41.56%), liver (51.60%), lungs (6.16%) and kidneys (0.53%). After 6 hours of administration, the nanoparticles were mainly accumulated in liver (76.33%), lungs (11.86%) and kidneys (2.23%) [86].
Table 2, presents the biodistribution of AuNPs at 24 hours after IV administration in rats and can be observed that in the case of ultrasmall AuNPs, a very high concentration was present in liver, compared to AuNPs with higher dimensions. Also, the lung represents the primary entry route for airborne particles into the human body. It has been reported that, aerosolic NPs tend to aggregate and form structures of several hundred nm in diameter, changing the physico-chemical properties and interaction with cells. For example, Durantie E. and co-workers compared single AuNPs with aggregated AuNPs with hydrodynamic diameter of 32 and 106 nm, respectively. A 3D lung model was used and exposures were performed by aerosolization of the particles. No apparently harmful effects of single and aggregated AuNPs were observed using lactate dehydrogenase assay; also, the cell layer integrity was not impaired. The bio-distribution revealed that the majority of the AuNPs, single or aggregated, were inside the cells, and only a minor fraction (less than 5%) was found on the basolateral side. In the case of translocation rate, no significant difference was observed. However, aggregated AuNPs showed a significantly faster cellular uptake than single AuNPs at the first time point – 4 h [87].
Table 2. Biodistribution of AuNPs at 24 h after IV administration to rats, expressed as % of the given dose [88, 89].

4. Conclusions and Perspectives
AuNPs can be synthesized in a variety of shapes and sizes, can be conjugated with various coating agents to tailor their properties, and can also be used as core or shell for hybrid nanoparticles, to obtain different types of nanosystems with various applications. In the case of biomedical imaging, AuNPs are preferred because of their advantages such as large absorption in the near-infrared window for photothermal therapy and selective accumulation at sites of interest through the EPR effect. Due to these properties, along with their biocompatibility and low toxicity, AuNPs have led to an excellent performance in recent in vivo studies and clinical trials. Also, side effects of conventional drugs have been minimized by conjugation with AuNPs.
Future studies tend to focus in demonstrating the idea that AuNPs could be used as next generation theranostic agents. The concept of theranostics with multifunctional AuNPs has a great potential to enhance the medical area towards personalized medicine [90]. Also, another new dimension in the field of medicine refers to the AuNPs used as antiangiogenic agents in cancer therapy [91, 92].
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors declare no acknowledgments.
References
1. Grigore, M.E.; Biscu, E.; Holban, A.; Gestal, M.; Grumezescu, A. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals, 2016, Volume 9, Issue 4, pp. 75. https://doi.org/10.3390/ph9040075
2. Grigore, M. Organic and inorganic nano-systems used in cancer treatment. J. Med. Res. Healt Educ, 2017, Volume 1, Issue 1.
3. Singh, P.; Pandit, S.; Mokkapati, V. R. S. S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci., 2018, Volume 19, Issue 7, pp. 1979. https://doi.org/10.3390/ijms19071979.
4. Huang, H.-C.; Ramos, J.; Grandhi, T. S. P.; Potta, T.; Rege, K. Gold nanoparticles in cancer imaging and therapeutics. Nano Life, 2010, Volume 01, Issue 03n04, pp. 289-307.
5. Dykman, L.; Khlebtsov, N. Optical Properties of Gold Nanoparticles In: Gold nanoparticles in biomedical applications, 1st ed; CRC Press, 2017, pp. 7.
6. World Health Organization, Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer. 2018 (accessed on 25th July 2019).
7. Wang, S.; Lu, G. Applications of Gold Nanoparticles in Cancer Imaging and Treatment, in Noble and Precious Metals-Properties, Nanoscale Effects and Applications, IntechOpen, 2017, http://dx.doi.org/10.5772/intechopen.70901
8. Maity, R.; Chatterjee, M.; Banerjee, A.; Das, A.; Mishra, R.; Mazumder, S.; Chanda, N. Gold nanoparticle-assisted enhancement in the anti-cancer properties of theaflavin against human ovarian cancer cells. Mat Sci Eng C, 2019, Volume 104, pp.109909. https://doi.org/10.1016/j.msec.2019.109909
9. Kohout, C.; Santi, C.; Polito, L. Anisotropic Gold Nanoparticles in Biomedical Applications. Int. J. Mol. Sci., 2018, Volume 19, Issue 11, pp. 3385. https://doi.org/10.3390/ijms19113385
10. Lara-Cruz, C.; Jiménez-Salazar, J. E.; Arteaga, M.; Arredondo, M.; Ramón-Gallegos, E.; Batina, N.; Damián-Matsumura. Gold nanoparticle uptake is enhanced by estradiol in MCF-7 breast cancer cells. Int. J. NanoMed., 2019, Volume 14, pp. 2705-2718. https://doi.org/ 10.2147/IJN.S196683
11. Chhour, P.; Naha, P. C.; Cheheltani, R.; Benardo, B.; Mian, S.; Cormode, D. P. Gold Nanoparticles for Biomedical Applications: Synthesis and In Vitro Evaluation. In book: Nanomaterials in Pharmacology, 1st ed; Lu, Z.R.m Sakuma, S., Eds; Humana Press: New York, 2016; pp. 87-111. https://doi.org/10.1007/978-1-4939-3121-7
12. Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T. C. Recent advances in gold nanoparticles for biomedical applications: from hybrid structures to multi-functionality. J Mater Chem B, 2019, Volume 7, Issue 22, pp.3480-3496. https://doi.org/10.1039/c9tb00557a.
13. García, M.; Bouzas, V.; Carmona, N. Synthesis of gold nanorods for biomedical applications. in Bonsai Project Symposium: Breakthroughs in Nanoparticles for Bio-Imaging. AIP Conference Proceedings (1275), 2010.
14. Freitas de Freitas, L.; Varca, G.; dos Santos Batista, J.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomater, 2018, Volume 8, Issue 11, pp.939. https://doi.org/10.3390/nano8110939
15. Sahoo, G.P.; Basu, S.; Samanta, S.; Misra, A. Microwave-assisted synthesis of anisotropic gold nanocrystals in polymer matrix and their catalytic activities. J. Exp. Nanosci., 2015, Volume 10, Issue 9, pp.690-702. https://doi.org/10.1080/17458080.2013.877163
16. Noroozi, M.; Zakaria, A.; Moksin, M. M.; Wahab, Z. A.; Abedini, A. Green formation of spherical and dendritic silver nanostructures under microwave irradiation without reducing agent. International journal of molecular sciences. Int J Mol Sci., 2012, Volume 13, Issue 7, pp.8086-8096. https://doi.org/10.3390/ijms13078086
17. Ngo, V.K.T.; Nguyen, D. G.; Huynh, T. P.; Lam, Q. V. A low cost technique for synthesis of gold nanoparticles using microwave heating and its application in signal amplification for detecting Escherichia ColiO157:H7 bacteria. ANSN, 2016, Volume 7, Issue 3, pp.035016. https://doi.org/10.1088/2043-6262/7/3/035016
18. Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H. K.; Modi, T.; Aguilar, Z. P.; Lawrenz, M. B. Gold nanoparticles: various methods of synthesis and antibacterial applications. Front. Biosci, 2014, Volume 19, pp.1320-44.
19. Shah, K.W.; Zheng, L. Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)trimethoxysilane. Mater, 2019, Volume 12, Issue 10, pp. 1680. https://doi.org/10.3390/ma12101680.
20. Al-Yasiri, A.; Khoobchandani, M.; Cutler, C. S.; Watkinson, L.; Carmack, T.; Smith, C. J.; Katti, K. V. Mangiferin functionalized radioactive gold nanoparticles (MGF-198 AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Transactions, 2017, Volume 46, Issue 42, pp.14561-14571. https://doi.org/10.1039/C7DT00383H
21. Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol, 2016, Volume 6, Issue 4, pp.525-2161. https://doi.org/10.4172/2161-0525.100038
22. Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis – A prospective review on drug delivery aspect. OpenNano, 2017, Volume 2, pp.37-46. https://doi.org/10.1016/j.onano.2017.07.001
23. Jameel, Z.N. Synthesis of The gold Nanoparticles with Novel Shape via Chemical Process and Evaluating The structural, Morphological and Optical Properties. Energy Procedia, 2017, Volume 119, pp.236-241. https://doi.org/10.1016/j.egypro.2017.07.075
24. Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep., 2018, Volume 8, Issue 1, pp.4589. https://doi.org/10.1038/s41598-018-22976-5
25. Sorescu, A. A.; Nuţă, A.; Ion, R. M.; Niţu, S. G.; Radu, N.; Teodorescu, S. Complex nanoconjugate materials obtained from eco-friendly gold and silver nanoparticles and zinc phthalocyanine derivatives. In Advanced Topics in Optoelectronics, Microelectronics, and Nanotechnologies IX, Romania: Constanta, 2018 p. 109770H.
26. Thirumurugan, A.; Ramachandran, S.; Tomy, N. A.; Jiflin, G. J.; Rajagomathi, G. Biological synthesis of gold nanoparticles by Bacillus subtilis and evaluation of increased antimicrobial activity against clinical isolates. Korean J Chem Eng, 2012, Volume 29, Issue 12, pp.1761-1765. https://doi.org/10.1007/s11814-012-0055-7
27. Maliszewska, I.; Aniszkiewicz, L.; Sadowski, Z. Biological Synthesis of Gold Nanostructures Using the Extract of Trichoderma koningii. Acta Physica Polonica, A, 2009, Volume 116, Issue Supplement, pp.S-163-S-165. https://doi.org/10.12693/APhysPolA.116.S-163
28. Madhusudhan, A.; Bandi, R. Microwave-irradiated green synthesis of gold nanoparticles for catalytic and anti-bacterial activity. JAST, 2017, Volume 08, pp.1-9. https://doi:.org/10.1186/s40543-017-0121-1.
29. Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H. K.; Modi, T.; Aguilar, Z. P.; Lawrenz, M. B. Gold nanoparticles: various methods of synthesis and antibacterial applications. Front Biosci, 2014, Volume 19, Issue 8, pp.1320-1344.
30. Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2017, Volume 9, Issue 4, https://doi.org/10.1002/wnan.1449
31. Garcia Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D. A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem Photobiol Sci, 2018, Volume 17, Issue 11, pp.1534-1552. https://doi.org/10.1039/C8PP00271A
32. Nune, S.K.; Gunda, P.; Thallapally, P. K.; Lin, Y. Y.; Laird Forrest, M.; Berkland, C. J. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv, 2009. Volume 6, Issue 11, pp.1175-1194. https://doi.org/ 10.1517/17425240903229031
33. Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angewandte Chem Int Ed, 2014, Volume 53, Issue 7, pp. 1756-1789. https://doi.org/10.1002/anie.201300441.
34. Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res., 2010, Volume 1, Issue 1, pp. 13-28. https://doi.org/10.1016/j.jare.2010.02.002.
35. Huang, X.; El-Sayed, I. H.; Qian, W.; El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. JACS, 2006, Volume 128, Issue 6, pp.2115-2120. https://doi.org/10.1021/ja057254a.
36. Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: a focus on micelles. Contrast Media Mol Imaging, 2014, Volume 9, Issue 1, pp.37-52. https://doi.org/10.1002/cmmi.1551.
37. Xi, D.S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. Rsc Adv, 2012, Volume 2, Issue 33, pp.12515-12524. https://doi.org/10.1039/C2RA21263C
38. Cole, L.E.; Ross, R. D.; Tilley, J. M; Vargo-Gogola, T.; Roeder, R. K. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomed, 2015, Volume 10, Issue 2, pp.321-341. https://doi.org/10.2217/nnm.14.171
39. Bulte, J.W.; Modo, M. Introduction: The Emergence of Nanoparticles as Imaging Platform in Biomedicine In: Nanoparticles in biomedical imaging: emerging technologies and applications. Springer Science & Business Media, 2007; Volume 3, pp. 5-8.
40. Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale, 2012, Volume 4, Issue 6, pp.1871-1880. https://doi.org/10.1039/c1nr11188d
41. Xu, C.; Tung, G.A.; Sun, S. Size and Concentration Effect of Gold Nanoparticles on X-ray Attenuation As Measured on Computed Tomography. Chem Mater, 2008, Volume 20, Issue 13, pp.4167-4169. https://doi.org/10.1021/cm8008418.
42. Kim, T.; Lee, N.; Arifin, D. R.; Shats, I.; Janowski, M.; Walczak, P.; Bulte, J. W. In Vivo Micro-CT Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly-l-Lysine Nanocomplexes. Adv. Funct. Mater.,, 2017, Volume 27, Issue 3, pp.1604213-n/a. https://doi.org/10.1002/adfm.201604213.
43. Hainfeld, J.; Slatkin, D. N.; Focella, T. M.; Smilowitz, H. M. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol, 2006, Volume 79, Issue 939, pp.248-253. https://doi.org/10.1259/bjr/13169882
44. Kim, D.; Park, S.; Lee, J. H.; Jeong, Y. Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. JACS, 2007, Volume 129, Issue 24, pp.7661-7665. https://doi.org/10.1021/ja071471p
45. Cai, Q.-Y.; Kim, S. H.; Choi, K. S.; Kim, S. Y.; Byun, S. J.; Kim, K. W.; Yoon, K. H. Colloidal Gold Nanoparticles as a Blood-Pool Contrast Agent for X-ray Computed Tomography in Mice. Invest Radiol, 2007, Volume 42, Issue 12, pp. 797-806. http://doi.org/10.1097/RLI.0b013e31811ecdcd
46. Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Roux, S. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. JACS, 2008, Volume 130, Issue 18, pp.5908-5915. https://doi.org/10.1021/ja078176p.
47. Aziz, F.; Nazir, A. I.; Ahmad, A.; Bajwa, I.; Rehman, S. Z.; Diallo, A.; Khan, W. S. Novel route synthesis of porous and solid gold nanoparticles for investigating their comparative performance as contrast agent in computed tomography scan and effect on liver and kidney function. Int J Nanomedicine, 2017, Volume 12, pp.1555-1563. https://doi.org/10.2147/IJN.S127996.
48. Dreifuss, T.; Motiei, M.; Betzer, O.; Popovtzer, A.; Abourbeh, G.; Mishani, E.; Popovtzer, R. Glucose-functionalized gold nanoparticles as a metabolically targeted CT contrast agent for distinguishing tumors from non-malignant metabolically active processes. Proc. SPIE 10077, Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XIV, 100770I, 2017, San Francisco, California, United States https://doi.org/10.1117/12.2249850.
49. Kennedy, L.C.; Bickford, L. R.; Lewinski, N. A.; Coughlin, A. J.; Hu, Y.; Day, E. S.; Drezek, R.A. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small, 2011, Volume 7, Issue 2, pp.169-83. https://doi.org/10.1002/smll.201000134.
50. Riley, R.S.; Day, E.S. Gold nanoparticle‐mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2017, Volume 9, Issue 4, https://doi.org/10.1002/wnan.1449.
51. Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light‐Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo. Adv. Mater., 2017, Volume 29, Issue 6, https://doi.org/10.1002/adma.201604894.
52. Agarwal, A.; Huang, S. W.; O’donnell, M.; Day, K. C.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys., 2007, Volume 102, Issue 6, pp.064701. https://doi.org/10.1063/1.2777127.
53. Yang, Z.; Song, J.; Dai, Y.; Chen, J.; Wang, F.; Lin, L.; Fan, W. Self-Assembly of Semiconducting-Plasmonic Gold Nanoparticles with Enhanced Optical Property for Photoacoustic Imaging and Photothermal Therapy. Theranostics, 2017, Issue 7, Volume 8, pp.2177-2185. https://doi:.org/10.7150/thno.20545.
54. Taruttis, A.; Herzog, E.; Razansky, D.; Ntziachristos, V. Real-time imaging of cardiovascular dynamics and circulating gold nanorods with multispectral optoacoustic tomography. Optics Express, 2010, Volume 18, Issue 19, pp.19592-19602. https://doi.org/10.1364/OE.18.019592
55. Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomed, 2015, Volume 10, Issue 2, pp.299-320. https://doi.org/10.2217/nnm.14.169.
56. Pence, I.; Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev., 2016, Volume 45, Issue 7, pp.1958-1979. https://doi.org/10.1039/c5cs00581g.
57. Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics — From in-vitro biofluid assays to in-vivo cancer detection. Adv Drug Deliv Rev, 2015, Volume 89, Issue Supplement C, pp.121-134. https://doi.org/10.1016/j.addr.2015.03.009
58. Andreou, C.; Kishore, S.A.; Kircher, M.F. Surface-Enhanced Raman Spectroscopy: A New Modality for Cancer Imaging. J Nuclear Med, 2015, Volume 56, Issue 9, pp.1295-1299. https://doi.org/10.2967/jnumed.115.158196
59. Zavaleta, C.L.; Smith, B.R.; Walton, I.; Doering, W.; Davis, G.; Shojaei, B.; Gambhir, S.S. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. PNAS, 2009, Volume 106, Issue 32, pp.13511-13516. https://doi.org/10.1073/pnas.0813327106.
60. Ursu, E.-L.; Doroftei, F.; Peptanariu, D.; Pinteala, M.; Rotaru, A. DNA-assisted decoration of single-walled carbon nanotubes with gold nanoparticles for applications in surface-enhanced Raman scattering imaging of cells. J Nanopart Res, 2017, Volume 19, Issue 5, pp.181. https://doi.org/10.1007/s11051-017-3876-9.
61. Li, J.; Gupta, S.; Li, C. Research perspectives: gold nanoparticles in cancer theranostics. Quant Imaging Med Surg, 2013, Volume 3, Issue 6, pp.284. https://doi.org/10.3978/j.issn.2223-4292.2013.12.02.
62. Lim, Z.-Z.J.; Li, J.E.; Ng, C.T.; Yung, L.Y.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta Pharmacol Sin., 2011, Volume 32, Issue 8, pp.983-990. https://doi.org/10.1038/aps.2011.82.
63. Yang, C.; Bromma, K.; Di Ciano-Oliveira, C.; Zafarana, G.; van Prooijen, M.; Chithrani, D.B. Gold nanoparticle mediated combined cancer therapy. Cancer Nanotechnol., 2018, Volume 9, Issue 1, pp.4. https://doi.org/10.1186/s12645-018-0039-3
64. Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol Sci Appl, 2008, Volume 1, pp.17-32, https://doi.org/10.2147/NSA.S3788.
65. Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol, 2012, Volume 85, Issue 1010, pp.101-113. https://doi.org/10.1259/bjr/59448833.
66. Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol Pharm, 2019, Volume 16, Issue 1, pp.1-23. https://doi.org/ 10.1021/acs.molpharmaceut.8b00810
67. Shivani, V.; Utreja, P.; Rahman, M.; Kumar, L. Gold Nanoparticles and their Applications in Cancer Treatment. Curr. Nanomed., 2018, Volume 8, Issue 3, pp.184-201. https://doi.org/10.2174/2468187308666180312130055
68. Vines, J.B.; Lim, D. J.; Vines, J. B.; Yoon, J.H.; Ryu, N.E. Gold Nanoparticles for Photothermal Cancer Therapy. Front Chem, 2019, Volume 7, pp.167, https://doi.org/10.3389/fchem.2019.00167
69. Ma, X.; Hui, H.; Jin, Y.; Dong, D.; Liang, X.; Yang, X.; Tian, J. Enhanced immunotherapy of SM5-1 in hepatocellular carcinoma by conjugating with gold nanoparticles and its in vivo bioluminescence tomographic evaluation. Biomater, 2016, Volume 87, pp.46-56. https://doi.org/10.1016/j.biomaterials.2016.02.007
70. Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci. Rep., 2018, Volume 8, Issue 1, pp.3815. https://doi.org/10.1038/s41598-018-22172-5.
71. Wang, F.; Wang, Y. C.; Dou, S.; Xiong, M. H.; Sun, T.M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS nano, 2011, Volume 5, Issue 5, pp.3679-3692. https://doi.org/10.1021/nn200007z.
72. Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Wang, Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep., 2018, Volume 8, Issue 1, pp.2907. https://doi.org/10.1038/s41598-018-21331-y
73. Park, H.; Tsutsumi, H.; Mihara, H. Cell-selective intracellular drug delivery using doxorubicin and α-helical peptides conjugated to gold nanoparticles. Biomater., 2014, Volume 35, Issue 10, pp.3480-3487. https://doi.org/10.1016/j.biomaterials.2013.12.094
74. Asadishad, B.; Vossoughi, M.; Alemzadeh, I. Folate-receptor-targeted delivery of doxorubicin using polyethylene glycol-functionalized gold nanoparticles. Ind Eng Chem Res, 2010, Volume 49, Issue 4, pp.1958-1963. https://doi.org/10.1021/ie9011479.
75. Chen, Y.-H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Wu, C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm., 2007, Volume 4, Issue 5, pp.713-722. https://doi.org/10.1021/mp060132k
76. Li, J.; Wang, X.; Wang, C.; Chen, B.; Dai, Y.; Zhang, R.; Fu, D. The Enhancement Effect of Gold Nanoparticles in Drug Delivery and as Biomarkers of Drug‐Resistant Cancer Cells. ChemMedChem, 2007, Volume 2, Issue 3, pp.374-378. https://doi.org/10.1002/cmdc.200600264.
77. Dreaden, E.C.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M. A. Tamoxifen-poly(ethylene glycol)-thiol gold nanoparticle conjugates: enhanced potency and selective delivery for breast cancer treatment. Bioconjug Chem, 2009, Volume 20, Issue 12, pp.2247-53. https://doi.org/10.1021/bc9002212.
78. Pandey, S.; Mewada, A.; Thakur, M.; Shah, R.; Oza, G.; Sharon, M. Biogenic gold nanoparticles as fotillas to fire berberine hydrochloride using folic acid as molecular road map. Mat Sci Eng C, 2013, Volume 33, Issue 7, pp.3716-3722. https://doi.org/10.1016/j.msec.2013.05.007.
79. Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Yaszemski, M.J. Targeted Delivery of Gemcitabine to Pancreatic Adenocarcinoma Using Cetuximab as a Targeting Agent. Cancer Res., 2008, Volume 68, Issue 6, pp.1970-1978. https://doi.org/10.1158/0008-5472.CAN-07-6102.
80. Azzam, E.; Morsy, S.M.I. Enhancement of the Antitumour Activity for the Synthesised Dodecylcysteine Surfactant using Gold Nanoparticles. J Surfactants Deterg, 2008, Volume 11, pp.195-199. https://doi.org/10.1007/s11743-008-1072-8.
81. Schmid, G.; Kreyling, W.G.; Simon, U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch Toxicol, 2017, Volume 91, Issue 9, pp.3011-3037. https://doi.org/10.1007/s00204-017-2016-8.
82. Harper, S.; Usenko, C.; Hutchison, J.E.; Maddux, B.L.S.; Tanguay, R.L. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalisation and route of exposure.J. Exp. Nanosci., 2008, Volume 3, Issue 3, pp.195-206. https://doi.org/10.1080/17458080802378953.
83. Xia, Q.; Li, H.; Xiao, K. Factors affecting the pharmacokinetics, biodistribution and toxicity of gold nanoparticles in drug delivery. Curr Drug Metab, 2016, Volume 17, Issue 9, pp.849-861.
84. Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep., 2017, Volume 7, Issue 1, pp.3303.
85. Aborig, M.; Malik, P.R.; Nambiar, S.; Chelle, P.; Darko, J.; Mutsaers, A.; Wettig, S. Biodistribution and Physiologically-Based Pharmacokinetic Modeling of Gold Nanoparticles in Mice with Interspecies Extrapolation. Pharmaceutics, 2019, Volume 11, Issue 4, pp.179. https://doi.org/10.3390/pharmaceutics11040179.
86. Le, Q.L.; Do, T.P.L.; Nguyen, H.P.U.; Nguyen, Q.H. Biodistribution of gold nanoparticles synthesized by γ-irradiation after intravenous administration in mice. ANSN, 2014, Volume 5, Issue 2, pp.025009. https://doi.org/10.1088/2043-6262/5/2/025009.
87. Durantie, E.; Vanhecke, D.; Rodriguez-Lorenzo, L.; Delhaes, F.; Balog, S.; Septiadi, D.; Rothen-Rutishauser, B. Biodistribution of single and aggregated gold nanoparticles exposed to the human lung epithelial tissue barrier at the air-liquid interface. Part Fibre Toxicol, 2017, Volume 14, Issue 1, pp.49-49. https://doi.org/10.1186/s12989-017-0231-3.
88. Khlebtsov, N., Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev, 2011, Volume 40, Issue 3, pp.1647-71. https://doi.org/ 10.1039/C0CS00018C.
89. Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of gold nanoparticles. Nanotechnol Russ, 2011, Volume 6, pp.17-42. https://doi.org/10.1134/S1995078011010101.
90. Dreifuss, T.; Barnoy, E.; Motiei, M.; Popovtzer, R. Theranostic gold nanoparticles for CT imaging, in Design and Applications of Nanoparticles in Biomedical Imaging; Bulte, J., Modo, M., Eds.; Springer: Cham, 2017, pp.403-427. https://doi.org/10.1007/978-3-319-42169-8_19
91. Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics, 2018, Volume 8, Issue 2, pp.533-548. https://doi.org/10.7150/thno.21674.
92. Saeed, B.A.; Lim, V.; Yusof, N.A.; Khor, K. Z.; Rahman, H.S.; Samad, N.A. Antiangiogenic properties of nanoparticles: a systematic review. Int J Nanomedicine, 2019, Volume 14, pp.5135. https://doi.org/10.2147/IJN.S199974.