Materials International 2019, 1, 1, 0002 -0012, https://doi.org/10.33263/Materials11.002012
Denisa-Alexandra Florea 1, Ecaterina Andronescu 1, Alexandru Mihai Grumezescu 1,*
1 Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Materials Science, Politehnica University of Bucharest, 060042 Bucharest, Romania
* Correspondence: alexandru.grumezescu@upb.ro
Abstract: Currently, the transplant crisis is one of the main concerns in the healthcare systems all over the world, the lack of donors and the persons which are on the waiting list for a transplant being higher from year to year. The diseases at the bone tissue level are affecting about 75M in USA, Europe and Japan, the need of treatments in this field becoming clear. During the last decades, the USA spent more than 20 billion dollars on treatments for bone trauma and more than 300.000 spinal fusions were conducted only in 2005. Moreover, the International Osteoporosis Foundation stated that the number of hip fractures may increase by four by 2050. Therefore, considering the data reported for the last decades and the predictions made for the near future, there are two main directions which must be considered: the drawbacks of the current treatments and the economic impact of the available options. In this regards, Tissue Engineering is relatively new field in the regenerative medicine area, which aims to develop cost-effective alternatives for different diseases/trauma in order to restore the function of a tissue and to undertake the transplant crisis.
Keywords: biomaterials, bone tissue engineering, scaffolds.
| © 2019 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). |
1. Introduction
Currently, one of the most exploited fields in the regenerative medicine area is tissue engineering which aims to combine notions and concepts from engineering (impact, manufacturing, cost etc.) with biological facts (proteins, growth factors) by using the human body’s potential to repair/create new tissues. The main scope of tissue engineering (TE) is to undertake the transplant crisis, to restore the functions of the affected tissues or to propose suitable solutions for various diseases and/or trauma.
The 1980s was a remarkable decade in biomedical field due to Dr. Vacanti and Dr. Langer who suggested to construct and design polymeric scaffolds for cell delivery. The first paper in this regard was published in 1988, in Archives in Surgery, this being the moment when Tissue Engineering field was born. Starting from this point, TE field had a remarkable evolution, and nowadays, many companies around the world are developing tissue-engineered products which are used by physicians worldwide, especially at the skin, bone or cartilage level. However, some companies had to stop the production of such products because of high manufacturing costs [1-3].
At the beginning of
2018, the United Network for Organ Sharing (also known as UNOS) stated 115.445
people being on the waiting list for a lifesaving transplant, therefore the
need of tissue development is essential [4].
2. The main tools used in Tissue Engineering area
2.1. Scaffolds
Scaffolds are one of the main tools used in TE area being considered an artificial extracellular matrix (ECM) characterized by a specific shape which must provide mechanical support for the new tissue formation. The native extracellular matrix is composed of products secreted by cells, being a complex mixture of several proteins, growth factors and polysaccharides, which are meant to direct the cellular activity (proliferation, differentiation, migration) by providing the necessary biological, chemical and physical parameters, and to modulate the immune response.
A novel perspective in terms of ECM is represented by the proteins which are present in its structure, for example, fibronectin, which has a major role in supporting the cellular attachment and spreading, being a mediator in bacteria attachment as well – these facts are very important when it comes to cell-surface interactions [5-7].
Considering the fast development of tissue engineering, biological scaffolds which can deliver a functional microenvironment for cellular adhesion, growth and spreading, as well as having the ability to integrate in the required tissue have gained researchers attention in the last years. Therefore, various biologic scaffolds made of allogenic/xenogeneic ECM were used in order to rebuilt tissues such as skin, urinary bladder etc. However, it was established that the manufacturing process, the origin of the cells or the sterilization may affect the mechanical properties, degradation rate or the cytocompatibility of the scaffold [8].
However, many materials are used in order to create 3D matrices for tissue engineering, especially natural and synthetic polymers such as PLGA – poly(lactic-co-glycolic acid), silk, PCL – poly(caprolactone) or ceramics like tricalcium phosphate – TCP, hydroxyapatite – HA or composite biomaterials. The choice of the materials used as scaffolds always depends on the application [9].
2.2. Stem cells and growth factors
Starting from the fact that cells are capable to create tissues if they are cultured in certain conditions, TE is based on the idea to create them by combining cells, growth factors and 3D matrices with specific structures, surface chemistry, architectures etc.
Therefore, one of the most exploited type of cells used in the field is the stem cells class. A stem cell is usually classified as an undifferentiated cell which can be divided in copies of itself and it can differentiate in other cell types. They are grouped in two main categories:
- Adult stem cells
- Embryonic stem cells
Embryonic stem cells are derived from human/other species embryo and they present pluripotency which means that they can follow the differentiation process, being able to transform themselves in more than 200 types of cells under suitable conditions, while adult stem cells present a multipotent character – they can differentiate only in a restricted number of cell phenotypes [10].
As it was stated before, tissue engineering is exploiting the ability of the organism to recover after a trauma/disease. This ability is linked with the existence of stem cells populations within various tissues/organs in the human body which reacts in order to restore the function of the affected tissue/organ. However, bone marrow represents a key reservoir of hematopoietic (HSCs) and mesenchymal (MSCs) stem cells.
Recognized by scientists as blood forming cells, HSCs are found in mesodermal hemangioblasts, being responsible for the continuous renewal of blood elements. However, the most exploited category of stem cells in TE is the mesenchymal stem cells class which can be defined as cells derived from various connective tissues, being found in brain, heart, teeth, blood vessels, skeletal muscle etc.
The potential of MSCs to differentiate in a wide range of cell phenotypes such as osteoblasts, chondrocytes, myocytes etc. has gained a lot of attention in the last decades, especially in the last years, when recent studies suggested that they can differentiate in liver cells as well. However, even if they are used by scientists and physicians due to many advantages (such as ease of isolation, manipulability, proliferation rate or the ability to differentiate in the desired tissue under proper conditions), deeper research is required to confirm the liver cells differentiation theory and to establish their real potential [11].
From the beginning, the main principle of tissue engineering was based on the use of scaffolds, stem cells and growth factors, which are substances utilized in this field as signaling molecules, to direct the cells in order to migrate, proliferate or differentiate. Despite this, nowadays, the research is focused on developing growth factors-free alternatives because of their high cost [6].
3. Bone tissue engineering (BTE)- an overview
3.1. Bone tissue- structure and function
The bone tissue is one of the most complex organs in the human body being composed of three main components: hydroxyapatite (HA), which represents about 70% from the total percentage of the mineral phase, collagen-I – about 20%, water – 8% and other constituents such as magnesium, non-collagenous proteins (usually having the function of growth factors, in order to promote bone formation), lipids etc. Collagen-I is a very important component of the bone matrix, its structure having a crucial role in the mineralization process.
Collagen-I is present in various tissues in the human body, especially in skin, bones and tendons, having a triple helix conformation. The bone matrix appears before the mineralization process and in its structure, collagen exists as chains which are connected into fibrils. The fibrils are structured as parallel layers which are meant to support the crystal depositions between them [13].
The specialized cells involved in the osteogenesis (bone formation process) – osteoblasts, osteoclasts and osteocytes, are very complex and they are the main elements responsible for the regeneration capacity of the bone tissue. The potential of these cells is constantly investigated and exploited in order to develop materials which can direct their activity and accelerate the regeneration [13].
Even if bone is a dynamic tissue, there are many factors which can affect the integrity and function of the osseous tissue, such as diseases, fractures or various injuries. The most common diseases in this area are osteoporosis, osteoarthritis, Osteogenesis Imperfecta, sarcopenia or osteomyelitis, which can mainly lead to bone loss, bone break, slow osteogenesis process, and they affect the daily activities of a patient. Therefore, at the moment, the responsibility of an orthopaedic physician is to find a suitable product which can fulfil all the requirements requested by the damaged tissue [14, 15].
3.2. The need of BTE
Considering the data gained in the last years and the predictions made by scientists for the future, two main aspects must be considered: the current challenges in terms of medical issues and the economic impact of the available treatments.
Statistically, it was stated that, in the USA, the cost for treatments, in terms of bone trauma, which appeared as a result of diseases, accidents etc., was very high, being estimated at about 20 billion $ in 2003. Moreover, in 2005, the same amount of money was spent in the USA only on conducting 300.000 fusions.
Recently, the International Osteoporosis Foundation – IOF stated that osteoporosis affects more than 75M (million) people in the USA, Europe and Japan, leading to about 8M bone fractures/year, more than 6M being reported only for the USA. When it comes to the healing process of these fractures, about 8% don’t present a complete restoration because of bone loss, infections or incomplete vascularization. Furthermore, a recent report published by IOF in 2018, suggested that the number hip fractures caused by osteoporosis in Lebanon, Syria and Jordan is expected to be multiplied by 4 by 2050.
When it comes to disability, the rate reported for patient affected by osteoporosis is very high, being compared to the one reported for various types of cancers. The main issue at the moment is that the available treatments used for clinical practice, for example autografts and allografts have been associated over the time with poor integration, host rejection, disease transmission or other unfavorable reactions [9,14, 16].
3.3. Scaffold requirements
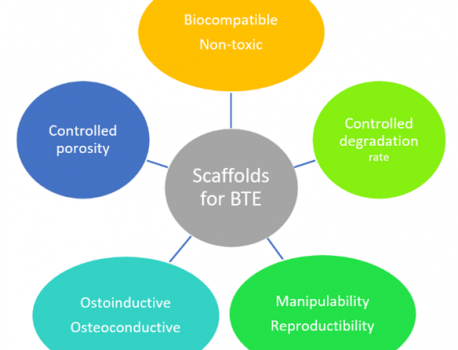
The success of osteogenesis is correlated with many factors including the design and manufacturing of the scaffolds (Figure 2). Therefore, there are several requirements which must be respected in order to sustain bone formation. In a general point of view, the materials used in the fabrication of a scaffold must be biocompatible and non-toxic, in order to maintain the cellular activity and to assure the completion of the angiogenesis process. They must possess a proper biodegradability rate, being able to provide the needed space for the newly formed bone.

The architecture of the scaffold must ensure a proper porous network capable to deliver mechanical support, similar to the native tissue. The porous network should provide a correct diffusion of the nutrients and O2 in order to ensure cell survival and it has to be well controlled to sustain the cellular attachment. Materials with increased porosity showed over the time deficient mechanical properties [9, 17, 19].
Osteoconduction and osteoinduction are key parameters, as well when it comes to projecting an ideal scaffold, because it must induce cellular attachment, spreading and differentiation in a specific direction [20].
In order to make these systems suitable for commercialization, it is very important to find a proper sterilization method – at industrial scale, to avoid multiple preparation procedures at the implantation time and to be able to be reproduced at a large scale by using cost-effective manufacturing techniques.
However, most of the scaffolds used in this field are fabricated from polymers, bioceramics or hybrids and they may be injectable or rigid, depending on the targeted application [19].
3.4. Scaffold manufacturing: current technologies
Currently, there are many technologies which are used in order to create the scaffold, but a novel approach in the field is the 3D printing process – rapid prototyping (RP) which aims to overcome the drawbacks reported for the conventional methods (Figure 3), such as: limited control of geometry, shape, architecture, or the use of different organic solvents which may affect the cellular viability [19].

Rapid prototyping is also known as additive manufacturing (AM) and allows the manufacturing of the 3D matrix by layer deposition and it can use liquids, solids or powders. These techniques offer a better control of the porosity, structure and architecture because of the equipment used. It starts from a 3D mathematical model and the entire equipment is composed of three main tools: a scanner which transmits the geometry gained from the source to a specialized software in an editable and readable format by a fabrication system and a manufacturing apparatus which transforms the data gained from the software in the desired product.
The advantages of using additive manufacturing in this field are numerous, including that there is no need of toxic organic solvents in the fabrication process (which leads to a higher biocompatibility, non-toxicity), a firm control of shape and structure or a well-ordered porosity. Moreover, nowadays, these techniques are used in the biomedical area, based on the data obtained for patients by using different imagining techniques such as MRI – Magnetic Resonance Imaging or CT – Computer Tomography [19].
Bioprinting has gained the attention of numerous research groups in the last years and it is a very exploited technique in tissue engineering area. Compared to conventional techniques, bioprinting uses living cells in the manufacturing process. The selection of the materials utilized as bioinks is a crucial step in the process – many bioinks are made of natural and synthetic polymers with a controlled viscosity [21].
4. The importance of calcium phosphates in BTE
Even if many materials were investigated over the time as scaffolds for bone tissue engineering, calcium phosphate (CaP) family is one of the most intensively studied group of ceramics because of its similarities when compared to the native tissue, in two main aspects: chemistry and structure. Moreover, Sun et al., stated that its degradation products are contributing to bone mineralization. Therefore, CaP-based scaffolds are usually made of hydroxyapatite, tricalcium phosphate (TCP) or biphasic CaP (BCP) – which is composed of HA and β-tricalcium phosphate).
CaP-based scaffolds can be fabricated using various methods, including 3D printing and gel casting. However, the controlled porosity is correlated with the process used for fabrication and with the thermal treatment applied for sinterization – the high temperature used in order to obtain a dense material affects the pore size and the mechanical integrity of the scaffold. For instance, Islam et al. suggested that the fracture stress and compressive modulus of porous composite scaffolds made of ceramics and polymers increased at higher temperatures [22].
An ideal scaffold should possess an interconnected pore network with pores that have sizes between 150-500 µm in diameter to permit the access of cells and growth factors and to promote vascularization. Therefore, the porosity is a key factor, being one of the main contributors to the osteocondutive capacity and bioactivity of a scaffold. This is one of the main drawbacks of these types of scaffolds, together with their compressive strength – for a HA scaffold a value of 30.2±6.0 MPa was reported, which is very high when compared to the compressive strength of spongy bone (4-12 MPA) and very low compared to the compact bone (around 150 MPa) [23, 24].
In
a recent study, Li et al.,
investigated the osteoinductive potential of the most common three materials
from CaP family used in the field – HA, TCP and BCP on bone marrow derived stem
cells (BMSC). After analyzing theese materials at different cellular
concentrations, it was observed that the hybrid BCP exhibited a better
expression of osteogenic markers compared to HA and TCP substrates, being
evident that the differentiation ability was linked with the composition of the
scaffold. Thus, it can be concluded that a combination of materials (from the
same class or even from different categories – e.g. polymers) may influence in
a positive way the osteoinductivity of the scaffold [25].
5. Hydroxyapatite- based scaffolds- properties and drawbacks
Considering that HA is an important member of CaP family, many studies have been made over the last decades in order to exploit this material by itself or in combination with various polymers. HA is used at the moment in various applications such as drug delivery systems (DDS), bioactive coatings – especially for metallic implants, scaffolds etc. due to its high biocompatibility.
Taking into account its formula (Ca10(OH)2(PO4)6) and its hexagonal structure, HA is one of the most stable CaP in physiological medium, being stable at pH between 4 and 12, being biocompatible for applications in hard tissue engineering area. The hexagonal structure is one of the main factors which offers a promising fatigue resistance to this inorganic compound [26-28].
However, the mechanical properties of HA were reported to be low, especially for scaffolds with macroporosity, therefore an increase in mechanical stability is necessary. As a consequence, research has been made to evaluate the behavior of HA doped with a range of reinforcement agents, for example different oxides (titanium, zirconium, magnesium or aluminium oxides), but the results obtained showed a significant decrease in bioactivity and biocompatibility [29-31].
Another issue reported for HA is the degradation rate correlated with its stability, which leads to a difficult resorption in the human body. As it was stated before, the controlled degradation rate is crucial for a scaffold used in bone tissue engineering area, therefore a HA-based composite is considered a viable alternative because it can be fabricated by adding a second phase which presents a higher degradation rate (e. g. silicates). In order to obtain an intimate contact between the implanted scaffold and the surrounding tissues, one approach in this regard is to obtain an optimal porosity. Therefore, many techniques are used to obtain this feature, including the freeze-casting method which is cost-effective and eco-friendly, this being attributed to the ice crystals utilized as pore-forming agents.
This technique was exploited by Jia et al., who created HA-silica (HA-SiO2) porous scaffolds with different silica ratios. A 3D matrix with oriented pore channels was obtained and characterized by various techniques, in order to evaluate the impact of silica on biodegradability and mechanical stability of the scaffold. After analyzing the samples with different amounts of SiO2, it was concluded that the SiO2 content had a negative impact on the compressive strength, but it improved the degradability rate and confirmed the importance of controlled porosity after SBF incubation. HA-5SiO2 (5% of SiO2 in composition) showed a faster deposition rate of the apatite layer on the surface when compared to simple HA, 2%SiO2 and 10%SiO2 [32].
The relation between the crystal grain size and the degradation rate was investigated by many research groups and it was observed that the size had a strong influence on both degradation and mechanical properties – nanoHA showed promising results compared to microHA (Lin and Chang, 2015).
This approach was evaluated as well in HA-based composites by a research group who prepared HA nanocrystallites in combination with N-acetylated chitosan (N-CS) and simple chitosan, which is a biopolymer widely used in tissue engineering field due to its antimicrobial nature, osteoconduction, minimal immune response and the ability to create porous matrices. Moreover, it is a promising material to be used in tissue engineering considering its structure, which is similar to the glycosaminoglycans, which are components of the ECM. Therefore, 3D porous scaffolds were developed and analyzed in terms of mechanical properties. The obtained scaffolds were compared with micron-sized HA used as control. The obtained results confirmed the presence of an open porous interconnected network for all the HA/CS and HA/N-CS scaffolds with a 90% porosity and pore sizes between 200µm – 700µm. The mechanical properties were higher for the scaffolds containing both polymer and nanoHA compared to the ones with micron-sized HA or with the polymeric matrix without inorganic part. The results confirmed the hypothesis that the grain size is correlated with the mechanical properties of the scaffold [33].
Moreover, the biocompatibility and osteoconductivity of HA/CS composites was demonstrated in numerous studies, including by Uswatta et al., who fabricated an injectable porous scaffold made from CS (0% and 2%) and nanoHA which were analyzed in terms of structural and functional parameters, mechanical properties, cytotoxicity and cellular response. The viability obtained for the composite scaffold was high, a few dead OB-6 cells were observed after 3 and 14 days of incubation. Superior cellular attachment and proliferation were observed, as well as a high confluency on the composite substrates, a remarkable increase being observed after 14 days on 2% CS nanoHA scaffolds, compared to 3 days. The favorable attachment was correlated with the high porosity of the substrate [34].
A new approach in terms of HA-based scaffolds starts from the idea that collagen is a constituent part of the bone tissue and it is well-known that HA-collagen scaffolds exhibit high biocompatibility, but poor mechanical properties and a fast degradation of collagen. Therefore, Sun et al., developed highly porous ultralong HA nanowires – UHANWs/Collagen – Col composite scaffolds with lengths of hundreds of µm, hierarchical porosity and various ratios of collagen, in order to evaluate the improvements made in terms of mechanical properties and cellular performance. The successful fabrication of the composite scaffold was determined by different characterization methods such as Scanning Electron Microscopy – SEM, X-ray Diffraction (XRD), and Transmission Electron Microscopy (TEM). The results showed that the flexibility of the nanowires allowed them to bend and to form a 3D fabric-like network. They were fully incorporated into the polymeric matrix, offering enhanced mechanical properties. Rat bone marrow-derived stem cells (r-BMSC) were used to investigate the cellular performance and it was concluded that, after 3 days of incubation, prominent cytoplasmic extensions were formed, being associated with the hierarchical porous structure of the scaffold. Considering these aspects, research is still needed to establish the cellular performance at various time moments and the exact improvement of the mechanical properties in terms of compressive strength, Young’s modulus and other relevant parameters [35].
As it was stated before, HA is a biomaterial which exhibits low osteoinduction and angiogenesis. These abilities together with bioactivity are crucial in terms of osteoblasts or stem cells stimulation. In order to solve these issues, the surface design and the chemical composition of the bulk material utilized as scaffold were taken into account by many scientists as parameters which can be improved for a better osteogenesis. [30]. Therefore, the incorporation of different elements such as Manganese (Mn), Zinc (Zn), Strontium (Sr), Chlorine (Cl), Titanium (Ti), Silicon (Si), Chromium (Cr), Potassium (K), Magnesium (Mg) or copper (Cu) into HA structure may have a positive impact on the osteogenic and angiogenic behavior of the material.
It is well-known that doping may induce various types of crystal defects, it changes the degradability, the biological response and it has a positive impact on the release of bioactive molecules from the biomaterial. For example, magnesium is one of the minor elements found in the native bone apatite and has a role in the metabolic activity of the human body, facts which lead to the idea that the Mg-doping could enhance the osteogenic character of HA. In terms of angiogenic potential, one of the well-known methods to induce the vascularization of a tissue is to add growth factors. One of the most common growth factors used in this regard is VEGF – vascular endothelial growth factor but its commercial use and clinical applications are limited due to its high costs. Recently, Kulanthaivel et al., suggested that the angiogenic and osteogenic activities of pure HA can be improved by doping with Co2+ and Mg2+ [36-38].
One of the main problems which can appear at the implantation site is the bacterial colonization and the appearance of infections which can lead to pain or other severe complications and results in the removal of the scaffold from the host. In order to control the biofilm formation, which is a five-steps process, it is very important to inhibit the bacteria attachment, to avoid the colonization and biofilm growth. The bacterial attachment is a very complex process due to bacterial-surface interactions. Therefore, in order to inhibit the biofilm development on the scaffold, a solution considered by scientist is doping with antibacterial agents, such as silver (Ag+) or zinc (Zn2+). However, in this regard, silver is the most recommended dopant, due to its controlled toxicity – low toxicity at low concentrations.
Polyvinyl alcohol (PVA) is another polymer which was studied during the years, together with hydroxyapatite, due to its suitable properties, such as biocompatibility, solubility, both thermal and chemical stability, biodegradability and non-toxicity. These properties support its use in various industries, such as cosmetic and pharmaceutical products, medical devices, packaging etc. Considering these aspects, Anjaneyulu et al., used Ag doped HA with PVA nanofibers (Ag@HA/PVA) obtained by an electrospinning process. They used AgNO3 as a dopant agent in the site of calcium (Ca), in order to evaluate the antimicrobial activity, bioactivity and in-vitro hemocompatibility. Two of the most common bacteria have been used for testing: S. aureus and E. coli. They were added in contact with the samples and incubated for 24h. The results confirmed the fact that the incorporation of Ag+ had a positive impact on the antimicrobial behavior against S. aureus and E. coli. However, a better result was observed in case of E. coli, and this might be attributed to the cell wall, which is less dense when compared to S. aureus. Moreover, the scaffolds presented a good hemolysis ratio, especially for the 5%Ag@HA/PVA samples (the highest amount used) and a proper deposition for the apatite layer after SBF immersion. Therefore, the potential of this material to exhibit antimicrobial properties in physiological medium should be further exploited [39 – 41].
6. Strontium (Sr)- Hydroxyapatite based composites for BTE
Starting from the idea that about 98% of the total amount of Strontium (Sr) found in the organism is present in the biological apatite, especially in places with a high metabolic activity, research has been made in drug delivery systems area and nowadays, two organic salts – Sr-ranelate and Sr-malonate are used as treatments for patients who are affected by osteoporosis, due to their potential to increase the proliferation rate of the bone-forming cells (osteoblasts) and to inhibit bone resorption (process conducted by osteoclasts) [42].
Considering the interest attributed to the incorporation of different elements in HA structure for various purposes, Sr represents a viable option to investigate, therefore Sr-substituted HA was developed, being able to successfully replace calcium (Ca) in the HA structure. The potential of this substitution was evaluated in DDS field by Lin et al. [43], who developed SrHA porous microspheres with distinct molar ratios (0.01 – Sr1HA, 0.03 – Sr3HA, 0.05 – Sr5HA) using the hydrothermal method. The aim of this research was to establish if the 3D nanostructured architecture of the materials had an influence on the drug loading and release profiles and to determine the effect on MG63 osteoblast- like cell-line and the angiogenic marker expression. Thus, it was confirmed that the incorporation did not affect the cellular viability, additionally the highest amount of Sr promoting the proliferation. For this set of samples, angiogenic expression was analyzed using Real-time polymerase chain reaction (RT-PCR). Results showed that VEGF angiogenic marker expression was higher in cells incubated with SrHA samples, compared to cells incubated with pure HA. This remarkable behavior can be exploited and further investigated, as being a key parameter in the treatment of big sized bone defects, where vascularization has to be induced by the use of growth factors [43].
Improvements in the biodegradability rate were observed and reported by Yatongchai et al., who analyzed the ion release profile for samples with two distinct substitutions – 5mole% Sr and 10mole% Sr. The results indicated that the release of P, Ca and Sr increased with the Sr content. Additionally, after 30 days of incubation in SBF, an accelerated deposition of the apatite layer was observed, compared to conventional HA and a proper viability was detected, being as good as for control samples, after the same amount of time in contact with MC-3T3-E osteoblasts [42].
In vivo studies were conducted by Chandran et al., in two different species: osteoporotic sheep and rat models. [44-45]. First, they fabricated microgranules of 10% SrHA (with the chemical formula Ca9Sr1(PO4)6(OH)2) and simple HA (used as control), in order to assess the osteogenic potential and the osteointegration speed. The obtained microgranules were characterized using XRD and SEM methods and were further implanted in nine osteoporotic rats, being evaluated by Micro-CT . After explantation, the newly bone formed in presence of 10%SrHA microgranules and presented a density comparable to the host native tissue [44]. The explants with HA microgranules exhibited a slight decrease in bone density, fact supporting the idea that such composition may be used in the treatment of the osteoporotic bones [44].
Starting from these results, further studies were conducted by Chandran et al., on osteoporotic sheep models, 10%SrHa and simple HA scaffolds being developed using the same method. MSCs derived from adipose tissue – ADMSCs – from the control sheep were used for in vitro tests. ADMSCs were cultured on the substrates and, after 7 days, a good cytocompatibility was observed. Additionally, the ALP (alkaline phosphatase) activity exhibited by cells incubated with 10%SrHA was comparable to control samples. The in vivo investigations done using Micro-CT, revealed that the release of Sr at the implantation site accelerated the osseointegration and bone formation [45].
Therefore, it can be concluded that the use of Sr has a positive impact on both osteoconduction, bone resorption and osteogenesis and further investigations should be made to asses its potential in terms of vascularization rate and antimicrobial activity.
Lei et al., suggested that the main components of the bone tissue, collagen and HA, might be replaced by chitosan and Sr-substituted HA. Therefore, the group developed SrHA/CS nanohybid scaffolds which were assessed in terms of morphology, structure, biocompatibility and the impact of Sr content on the osteoinductive capacity of HA. The samples were prepared as presented in Table 1. The SrHA/CS nanocomposite has been developed by using the freeze-drying technique, and a macroporous interconnected network with pore sizes varying from 100 to 400 µm was obtained.
Table 1. Sample preparation and labeling (Adapted from Lei et al., [46]).
| Sample | Molecular formula |
| HA | Ca10(PO4)6(OH)2 |
| Sr1HA | Ca9Sr1(PO4)6(OH)2 |
| Sr5HA | Ca5Sr5(PO4)6(OH)2 |
| Sr10HA | Sr10(PO4)6(OH)2 |
The obtained scaffolds [46] were evaluated in terms of cytotoxicity in contact with hBMSCs and it was concluded that the viability of the cells interacting with all scaffolds was high. The proliferation rates increased with the incubation time, and a well-spread morphology has been detected after 3 days of incubation. The samples were subjected to SEM investigations in order to asses the adhesion of hBMSCs and the images showed that the attachment point was inside the scaffold, the pores acting as channels for cells. The cellular phenotype was maintained [46]. However, different polymers should be studied as second phase and further investigation on the differentiation pathway of the hBMSCs can be investigated.
Considering the favorable response received in terms of cellular activity, further research may be conducted in order to establish the influence of Sr in CaP-based scaffolds, to avoid the complications which may appear after protein release, as a result of growth factors use in the human body. Moreover, it is well-known that Sr presents antibacterial properties, therefore, research may be conducted in this regard as well. The impact of Sr ions on the vascularization rate may be evaluated by the angiogenic marker expression in different composites. More in vivo studies are recommended as well, in order to see the true impact of the products on the living tissue.
7. Conclusions
The great potential of tissue-engineered products for bone implants has been demonstrated over the last years. However, there are many techniques which can improve the efficacity of these products, in order to decrease the costs, to improve the properties and to make them suitable for clinical use.
Even if there are loads of materials used in the field of bone tissue engineering, the most promising materials in terms of biocompatibility and cytotoxicity remains the CaP-based family. Therefore, the improvement of these materials by using the proper manufacturing method, in order to overcome the drawbacks is a main area of interest for scientists.
The incorporation of different elements in the HA structure represents a promising approach. Therefore, it was proved that the use of Sr as a substituted element in HA lattice may lead to promising results in terms of cellular viability, attachment, migration, proliferation on even differentiation. However, research is still needed in order to confirm its potential in terms of antibacterial activity or angiogenesis stimulation.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors declare no acknowledgments.
References
1. IntegraLife Brochure – Dermal Regeneration Template. Available Online: https://www.integralife.com/integra-lifesciences-launches-revize-revize-x-collagen-matrix-for-plastic-and-reconstructive-surgery/product/revizelaunch, (accessed on: 8th of January 2018).
2. Vacanti, C. A. The history of tissue engineering. J Cell Molec Med 2006, Volume 10, Issue 3, pp. 569-576, https://doi.org/10.1111/j.1582-4934.2006.tb00421.x.
3. Hüsing, B.; Bührlen, B; Gaisser, S. Human tissue engineered products: today’s markets and future prospects, Fraunhofer Institute for Systems and Innovation Research Karlsruhe, Germany 2003, Report no. 54, pp. 1 – 3.
4. UNOS, U. N. f. O. S. Facts about organ donation. Available Online: https://www.unos.org/donation/, (accessed on: 9th of January 2018).
5. Hernandez-Gordillo, V.; Chmielewski, J. Mimicking the extracellular matrix with functionalized, metal-assembled collagen peptide scaffolds. Biomat 2014, Volume 35, Issue 26, pp. 7363-7373, https://doi.org/10.1016/j.biomaterials.2014.05.019.
6. Furth, M.; Atala, A. Chapter 6: Tissue Engineering. Future Perspectives. In Principles of Tissue Engineering, 4th ed.; Lanza R., Langer R., Vacanti J., Eds.; Academic Press: Boston, USA, 2014; Volume 1, pp. 83-123, https://doi.org/10.1016/j.cis.2019.03.002.
7. Vacanti, J. P.; Vacanti, C. A. Chapter 1 – The History and Scope of Tissue Engineering. In Principles of Tissue Engineering, 4th ed.; Lanza R., Langer R., Vacanti J., Eds.; Academic Press: Boston, USA, 2014; Volume 1, pp. 3-8, https://doi.org/10.1016/B978-0-12-398358-9.00001-X.
8. Molnar, K.; Juriga, D.; Nagy, P. M.; Sinko, K.; Jedlovszky‐Hajdu, A.; Zrinyi, M. Electrospun poly (aspartic acid) gel scaffolds for artificial extracellular matrix. Polymer Int, 2014, Volume 63, Issue 9, pp.1608-1615, https://doi.org/10.1002/pi.4720.
9. Ng, J.; Spiller, K.; Bernhard, J.; Vunjak-Novakovic, G. Biomimetic approaches for bone tissue engineering. Tissue Eng Part B Rev, 2017, Volume 23, Issue 5, pp. 480-493, https://doi.org/10.1089/ten.teb.2016.0289.
10. Mummery, C.; van de Stolpe, A.; Roelen, B.; Clevers, H. Chapter 3 – What Are Stem Cells?. In Stem Cells, 2nd ed.; Academic Press: Boston, USA, 2014, Volume 1, pp. 53-68, ISBN: 987-0-12-411511-4.
11. Bethesda, M. Stem Cell Information Home Page. Available Online: https://stemcells.nih.gov/info/basics/4.htm (access on: 10th of January 2018).
12. Williams, J.K.; Andersson, K.-E. Regenerative pharmacology: recent developments and future perspectives, Regenerative medicine, 2016, Volume 11, Issue 8, pp. 859-870, https://doi.org/10.2217/rme-2016-0108.
13. Florea, D. A.; Grumezescu, A. M.;Titanium Implants with Modified Surface for Rapid Osseointegration, Germany, 2016, LAP Lambert Academic Publishing, pp. 1 – 10, ISBN: 978-3-659-87169-6.
14. IOF, I. O. F. Facts and statistics. Available Online: https://www.iofbonehealth.org/osteoporosis-musculoskeletal-disorders (accessed on: 10th of January 2018).
15. Fisher, J.; Reddi, A. Functional tissue engineering of bone: signals and scaffolds. Topics tissue eng, 2003, Volume 1, pp. 1-29.
16. Porter, J. R.; Ruckh, T. T.; Popat, K. C. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol progress, 2009, Volume 25, Issue 6, pp. 1539-1560, https://doi.org/10.1002/btpr.246.
17. Tang, D.; Tare, R. S.; Yang, L.-Y.; Williams, D. F.; Ou, K.-L.; Oreffo, R. O. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration, Biomat, 2016, Volume 83, pp. 363-382, https://doi.org/10.1016/j.biomaterials.2016.01.024.
18. Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv Colloid Interface Sci, 2017, Volume 249, pp.321-330, https://doi.org/10.1016/j.cis.2017.04.007.
19. Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl, 2017, Volume 78, pp.1246-1262, https://doi.org/10.1016/j.msec.2017.05.017.
20. Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magnesium-based scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl, 2017, Volume 71, pp.1253-1266, https://doi.org/10.1016/j.msec.2016.11.027.
21. Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; Dokmeci, M.R. 3D bioprinting for tissue and organ fabrication. Ann biomed eng, 2017, Volume 45, Issue 1, pp.148-163, https://doi.org/10.1007/s10439-016-1612-8.
22. Islam, M.S.; Todo, M. Effects of sintering temperature on the compressive mechanical properties of collagen/hydroxyapatite composite scaffolds for bone tissue engineering. Mater Lett, 2016, Volume 173, pp.231-234, https://doi.org/10.1016/j.matlet.2016.03.028.
23. Denry, I.; Kuhn, L. T. Design and Characterization of Calcium Phosphate Ceramic Scaffolds for Bone Tissue Engineering. Dent Mater, 2016, Volume 32, Issue 1, pp. 43–53, https://doi.org/10.1016/j.dental.2015.09.008.
24. Sun, H.; Yang, H.L. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin Med J (Eng), 2015, Volume 128, Issue 8, pp.1121, https://doi.org/10.4103/0366-6999.155121.
25. Li, Y.; Jiang, T.; Zheng, L.; Zhao, J. Osteogenic differentiation of mesenchymal stem cells (MSCs) induced by three calcium phosphate ceramic (CaP) powders: A comparative study. Mater Sci Eng C Mater Biol Appl, 2017, Volume 80, pp.296-300, https://doi.org/10.1016/j.msec.2017.05.145.
26. Michel, J.; Penna, M.; Kochen, J.; Cheung, H. Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int, 2015, Volume 2015, pp. 305217, http://dx.doi.org/10.1155/2015/305217.
27. Subhapradha, N.; Saravanan, D.; Selvamurugan, N.; Tsai, W.-B.; Srinivasan, N.; Murugesan, R.; Moorthi, A. Nanoceramics on osteoblast proliferation and differentiation in bone tissue engineering. Int J Biol Macromol, 2017, Volume 98, pp.67-76, https://doi.org/10.1016/j.ijbiomac.2017.01.089.
28. Roohani-Esfahani, S. I.; Newman, P.; Zreiqat, H. Design and fabrication of 3D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci rep, 2016, Volume 6, pp.19468, https://doi.org/10.1038/srep19468.
29. An, S.-H.; Matsumoto, T.; Miyajima, H.; Nakahira, A.; Kim, K.-H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent Mater, 2012, Volume 28, Issue 12, pp. 1221-1231, https://doi.org/10.1016/j.dental.2012.09.001.
30. Lin, K.; Chang, J. 1 – Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications, 1st ed.; Mucalo, M. Eds.; Woodhead Publishing Series in Biomaterials: Cambridge, United Kingdom, 2015, Volume 1, pp. 3-19.
31. Hickey, D. J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applications. Acta biomater, 2015, Volume 14, pp.175-184, https://doi.org/10.1016/j.actbio.2014.12.004.
32. Jia, Z.Q.; Guo, Z.X.; Chen, F.; Li, J.J.; Zhao, L.; Zhang, L. Microstructure, phase compositions and in-vitro evaluation of freeze casting hydroxyapatite-silica scaffolds. Ceramics Int, 2017, Volume 44, Issue 4, pp.3636-3643, https://doi.org/10.1016/j.ceramint.2017.11.114.
33. O’brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater Today, 2011, Volume 14, Issue 3, pp.88-95, https://doi.org/10.1016/S1369-7021(11)70058-X.
34. Uswatta, S. P.; Okeke, I. U.; Jayasuriya, A. C.; Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Mater Sci Eng C Mater Biol Appl, 2016, Volume 69, pp.505-512, https://doi.org/10.1016/j.msec.2016.06.089.
35. Sun, T. W.; Zhu, Y. J.; Chen, F.; Chen, F. F.; Jiang, Y. Y.; Zhang, Y. G.; Wu, J. Ultralong hydroxyapatite nanowires/collagen scaffolds with hierarchical porous structure, enhanced mechanical properties and excellent cellular attachment. Ceramics Int, 2017, Volume 43, Issue 17, pp.15747-15754, https://doi.org/10.1016/j.ceramint.2017.08.137.
36. Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceramics Int, 2015, Volume 41, Issue 9, pp.11323-11333, https://doi.org/10.1016/j.ceramint.2015.05.090.
37. Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; abu Osman, N.A. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceramics Int, 2014, Volume 40, Issue 4, pp.6021-6029, https://doi.org/10.1016/j.ceramint.2013.11.051.
38. Lu, J.; Wei, J.; Yan, Y.; Li, H.; Jia, J.; Wei, S.; Guo, H.; Xiao, T.; Liu, C. Preparation and preliminary cytocompatibility of magnesium doped apatite cement with degradability for bone regeneration. J Mater Sci Mater Med, 2011, Volume 22, Issue 3, pp.607-615, https://doi.org/10.1007/s10856-011-4228-4.
39. Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res, 2015, Volume 94, Issue 8, pp.1027-1034, https://doi.org/10.1177/0022034515587690.
40. Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: a review. Proc Inst Mech Eng H, 2014, Volume 228, Issue 10, pp.1083-1099, https://doi.org/10.1177/0954411914556137.
41. Anjaneyulu, U.; Priyadarshini, B.; Grace, A. N.; Vijayalakshmi, U. Fabrication and characterization of Ag doped hydroxyapatite-polyvinyl alcohol composite nanofibers and its in-vitro biological evaluations for bone tissue engineering applications. J Sol-Gel Sci Technol, 2017, Volume 81, Issue 3, pp.750-761, https://doi.org/10.1007/s10971-016-4243-5.
42. Yatongchai, C.; Placek, L.M.; Towler, M.R.; Wren, A.W. Effects of strontium substitution on bioactivity of hydroxyapatite. 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, New York, USA, 2015; IEE: Piscataway, New Jersey, USA, 2015, pp. 1-2, https://doi.org/10.1109/NEBEC.2015.7117137.
43. Lin, K.; Liu, P.; Wei, L.; Zou, Z.; Zhang, W.; Qian, Y.; Shen, Y.; Chang, J. Strontium substituted hydroxyapatite porous microspheres: surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem Eng J, 2013, Volume 222, pp. 49-59, https://doi.org/10.1016/j.cej.2013.02.037.
44. Chandran, S.; Vs, H.K.; Varma, H.K.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J Biomater Appl, 2016, Volume 31, Issue 4, pp.499-509, https://doi.org/10.1177/0885328216647197.
45. Chandran, S.; Shenoy, S. J.; Babu, S. S.; Nair, R. P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf B Biointerfaces, 2018, Volume 163, pp.346-354, https://doi.org/10.1016/j.colsurfb.2017.12.048.
46. Lei, Y.; Xu, Z.; Ke, Q.; Yin, W.; Chen, Y.; Zhang, C.; Guo, Y. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater Sci Eng C Mater Biol Appl, 2017, Volume 72, pp.134-142, https://doi.org/10.1016/j.msec.2016.11.063.